One Team for
All of Your Drug Development Needs!
One Team for all your drug development needs. From Hit-To-Lead to Preclinical and IND to Clinical Trials. Quality and on-time performance you can trust.
Read More
"Pre-Clinical Service"
Full Service Non-Clinical CRO with both IND-Enabling Packaging as well as Individual Services for all your Preclinical Needs.
Read More
"U.S. Regulatory and Clinical Trials"
Full Service Clinical Trials CRO for Early Phase in Maryland State, US with Extensive U.S. FDA Regulatory Affairs Services
Read MoreAbout
Who we are - One Team, One Goal!
Working together as one organization, QuBEST BIO and KCRN Research are a Global Full-Service CRO that services, manages, and consults on each step of your drug development pipeline with the quality support you need tailored to your study needs.
As the Nonclinical side of Operations based in South Korea, QuBEST BIO has maintained a track record of excellence in providing services within the Hit-To-Lead, Lead Optimization, and Preclinical stages enabling your novel ideas reach fruition.
Regulatory Affairs (Pre-IND and IND) and Early Phase Clinical Trials Operations KCRN Research based in Maryland next to U.S. FDA headquarters is your point of entry to making sure your compliance and studies are done to bring you within reach of your goals.
And our goal is to deliver highly effective and efficient results catered directly to your specific nonclinical, clinical, and regulatory needs by offering the experience you deserve with sensible and flexible solutions.
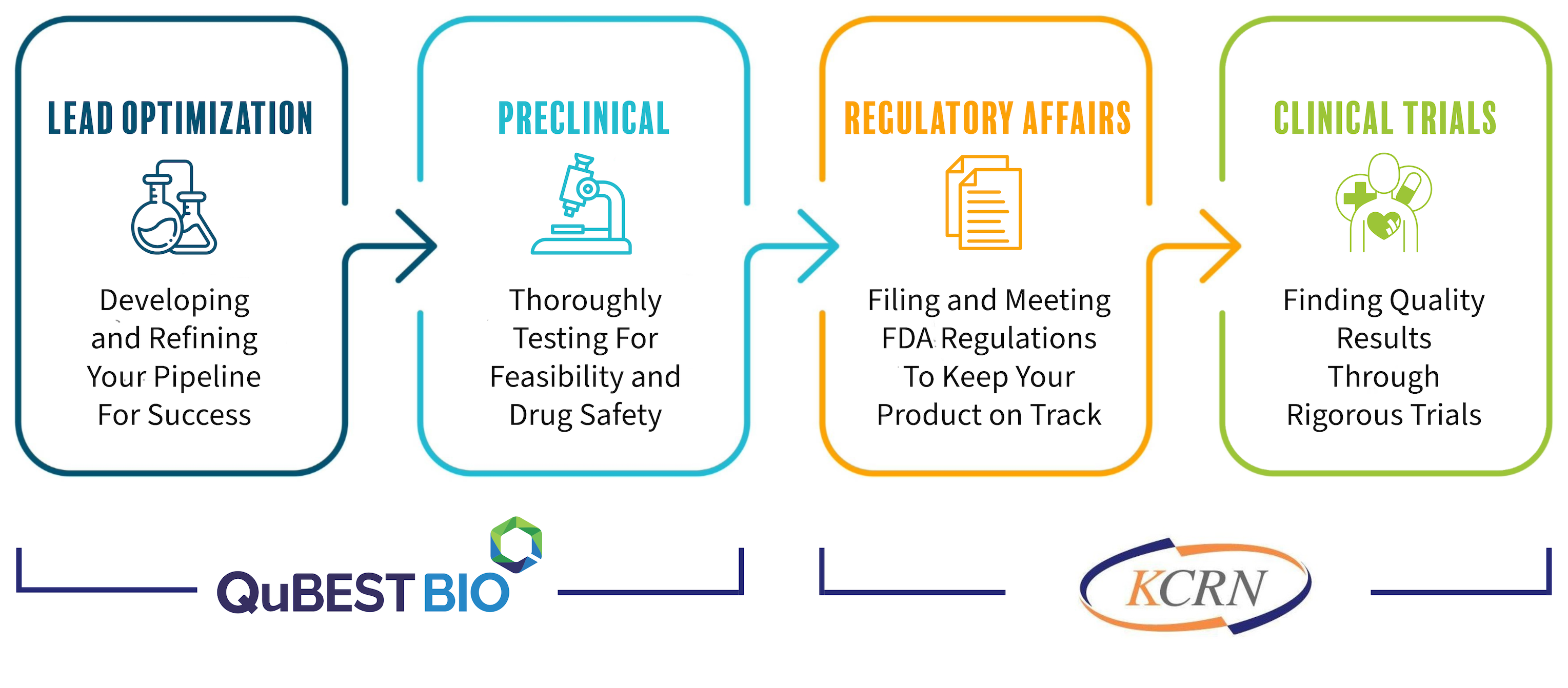
We are committed to developing and refining your pipeline with you as One Team.
Including both its Hit-To-Lead Services and Lead Optimization Services, QuBEST BIO is your best partner for making sure your drug development pipeline is handled on your terms and your goals between drug discovery and candidate selection.
-
Flexible design capabilities tailored to your study needs - Study support with a state-of-the-art bioanalysis facility.
- Rapid Screening capability from Hit-to-Lead to IND-enabling PK
- Strong background experience especially in the oncology, metabolic disorders, and inflammation therapeutic areas
- Fast and accurate data generation for today’s on-time needs

We handles all of your extensive and rigorous preclinical GLP and non-GLP needs through our experienced background.
Determining feasibility and safety of your drug is one of the most important early steps in determining whether it is the right choice for treatment.
- Highly experienced leadership in the Preclinical field from working both at QuBEST and before QuBEST
- Comprehensive internal and external GLP and non-GLP toxicology study support for many different sizes and scope
- Services in preliminary toxicology, general toxicology, genetic toxicology, DART and much more
- Flexibility and rapid turn-around for your study needs in dosing, administration, frequency, data & analysis

We are your guide to accomplishing that with experience over different sectors within the Regulartory Affairs
You can always count on KCRN Research and our RA team to being there for you to guide and prepare you through the process and help you achieve your goal of reaching IND approval and ready to start your clinical Trials.
- Full comprehensive GAP Analysis of your regulatory compliance
- RA services such as Pre-IND meetings, SAB Formation, Protocol Development and more
- IND filings, IND follow-ups and reporting
- Orphan Drug designation, Fast Track Designation and other incentives
- Getting your study compliant with the correct documentation to meet FDA regulations
Our RA team of professionals is your RA team. We are always on your side.

Our Clinical Trials operations is specifically built with efficiency in mind. Built directly to serve you.
KCRN Research prides itself on keeping true to its tenets within clinical research: reliable, on-time, and highly efficient solutions to your study needs. We work for you which means we work on your timing, your specific wants and needs, and with the ability to shift rapidly due to any rapid and/or unforeseen events or notices.
- Sponsor-centered operations though thorough effective communication between sponsors and team leads on every level as well as sponsor-specific goals and milestones
- Strong experience concentration within early phase clinical trials
- Effective partnerships and network with clinical sites both large and small throughout the U.S.
- Unique fixed-cost & incentive system to ensure collaboration and agreement geared towards efficiency for every step of the clinical trial process
- Utilizes own internal Clinical trials software package that emphasizes simplicity, security, and efficiency

"One Team For Your Pipeline!"
One team with QuBEST BIO and KCRN Research. And one team with you. We are built to with that principle in mind in order to provide you with the flexibility, efficiency, reasonable costs, and speed that you deserve.
Contact
Contact Us
Address
QuBEST BIO
#301, 16-25, Dongbaekjungang-ro 16beon-gil, Giheung-gu, Yongin-si, Gyeonggi-do, South Korea
KCRN Research, Inc.
12311 Middlebrook Rd Suite 200, Germantown, MD 20871
Email Us
Pre-Clinical: info@qubest.co.kr
US RA & Clinical Trials: info@kcrnresearch.com